
Thus, atoms of the same elements may not be identical in all the respects. For example, atoms of hydrogen may have atomic masses of 1 amu, 2 amu or 3 amu. Atoms made up of the same element can have different atomic masses:Ītoms that are made up of the same element and possess different atomic masses are called isotopes.

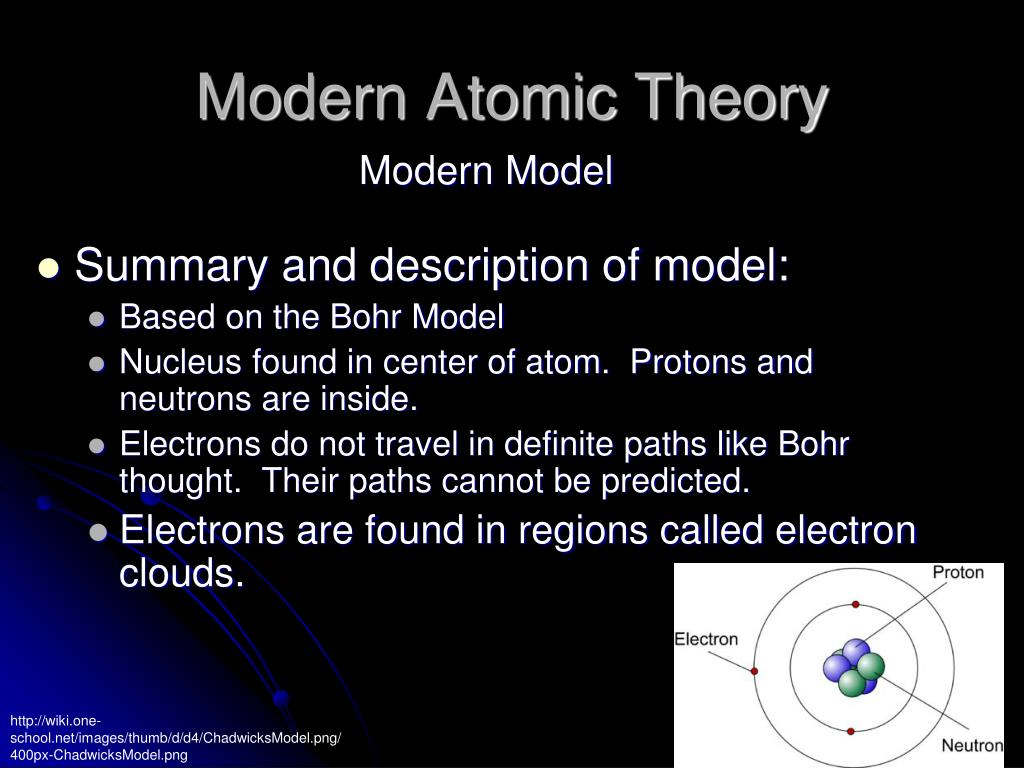
Though atom is made up of small particles such as electrons, neutrons and protons, yet the smallest particle that takes place in a chemical reaction is an atom. The smallest particle which takes part in a chemical reaction is the atom:.It has been found that an atom is made up of several small particles: electrons, neutrons and protons and thus, it possesses a complex structure. Ětom is no longer considered to be indivisible:.The main points of the modern atomic theory are: Yet, salient points of this theory have been retained as they satisfactorily explain the chemical laws of combination. The theory did not shed any light on the concept of binding force between various atoms and molecules which are responsible for the presence of three states of matter – solids, gases and liquids.Īs a result of the researches, made by different physicists and chemists, Dalton’s atomic theory has undergone radical changes.The theory makes no distinction between a compound or an element 's ultimate particles.The reason behind the combination of different atoms of the same or different elements to form molecules could not be explained by this theory.The theory also failed in explaining why atoms of different elements have different mass, size, valency, etc.This theory failed to explain the law of gaseous volume.The key limitations of the Dalton’s atomic theory are as follows:. At the beginning of the 20th century, the knowledge of the structure of the atom was revolutionized by brilliant researches conducted by Sir J.J Thomson, Neils Bohr, Rutherford and others. This theory was the very first step towards the internal structure of matter that provided the scientists a strong initiative during the 19th century to research matter.


 0 kommentar(er)
0 kommentar(er)
